In Georgia, the requirements for autoclave spore testing in tattoo studios are regulated by the Georgia Department of Public Health (DPH) and the state’s rules for tattooing. Autoclave spore testing is a critical practice to ensure that autoclaves are effectively sterilizing equipment used in tattooing procedures.

Governor Kemp signed into Law Senate Bill 214 which directs the Georgia Department of Public Health to adopt statewide Body Art regulations including the permitting of body artists. On March 6, 2023, the Department passed Chapter 511-3-8, Rules and Regulations for Body Art. Implementation of the Chapter will begin on October 6, 2023, six months after the effective posting date. Existing artists and studios will be given until October 6, 2024, a 12-month period, to meet the new requirements.
Woodhouse Laboratories can help you meet your testing requirements and comply with the regulations put into place by Governor Kemp.
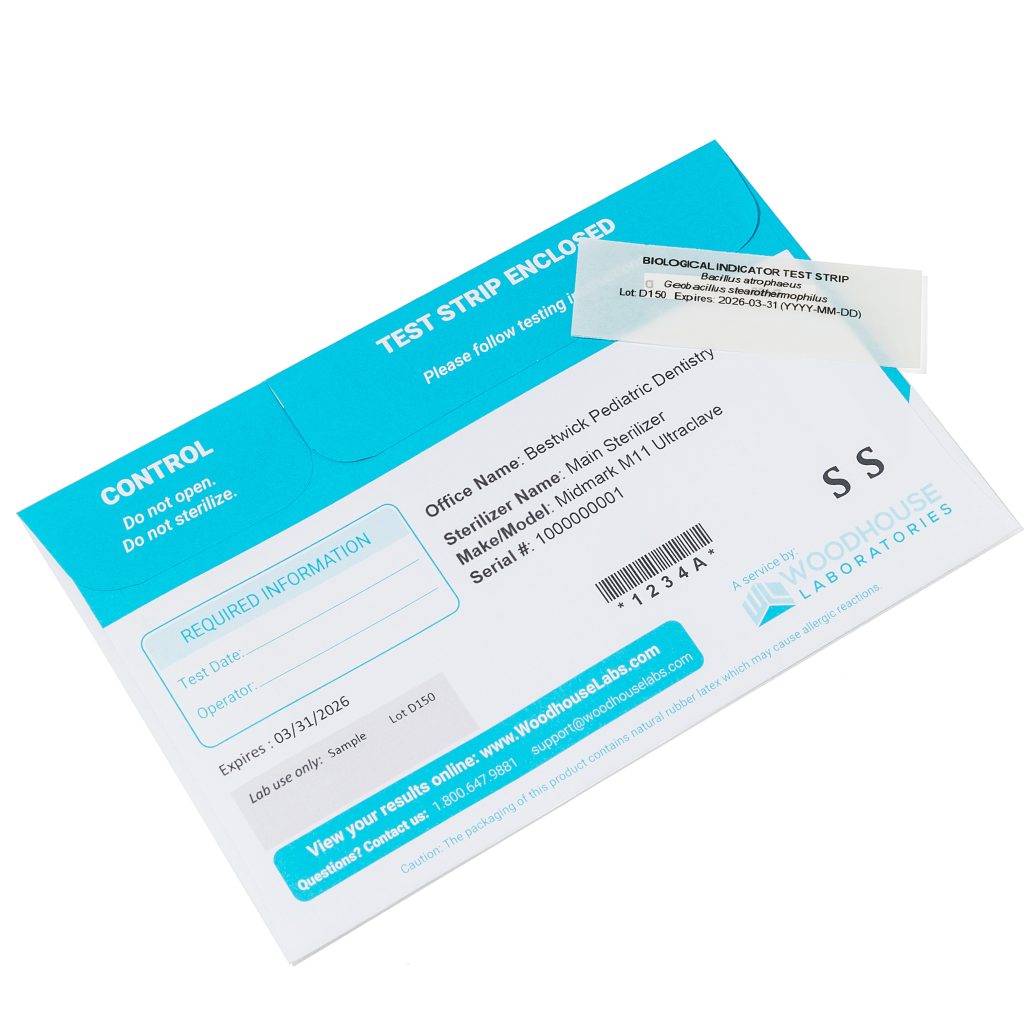
Spore tests shall be used at a minimum frequency of every 40 hours of operation of the
autoclave but not less than on a monthly basis unless the manufacturer specifies more frequent
monitoring. Records of the results must be kept for a minimum of three years. An independent
commercial testing laboratory contracted by the permit owner or body artist, or both shall
perform biological spore testing of the autoclave. A provision shall be included in the contract
with the commercial testing laboratory requiring the body art studio to notify the Health
Authority of any failure of the autoclave to eradicate all living organisms, including spores.
Upon notification of a positive microbiological monitoring report, the autoclave shall be
immediately checked for proper use and function and the permit owner shall cease use of the
autoclave immediately upon receipt of the positive report. Any items remaining bagged after
sterilization must be reprocessed and sterilized by a medical-grade autoclave approved for use
prior to return to service. A negative biological test and passing a Class 5 integrating indicator
must be achieved before the autoclave can be used again and the studio is reopened. The studio
shall have the option to obtain a properly functioning sterilizer with a negative biological report
in order to remain open or if the studio has more than one autoclave in operation, they may be
given approval to remain open. The Body Art Studio’s standard operation procedure should
include an emergency plan should an autoclave failure or malfunction occur.
For detailed guidance, tattoo studio owners should consult the Georgia Department of Public Health’s rules or local regulations regarding body art establishments.